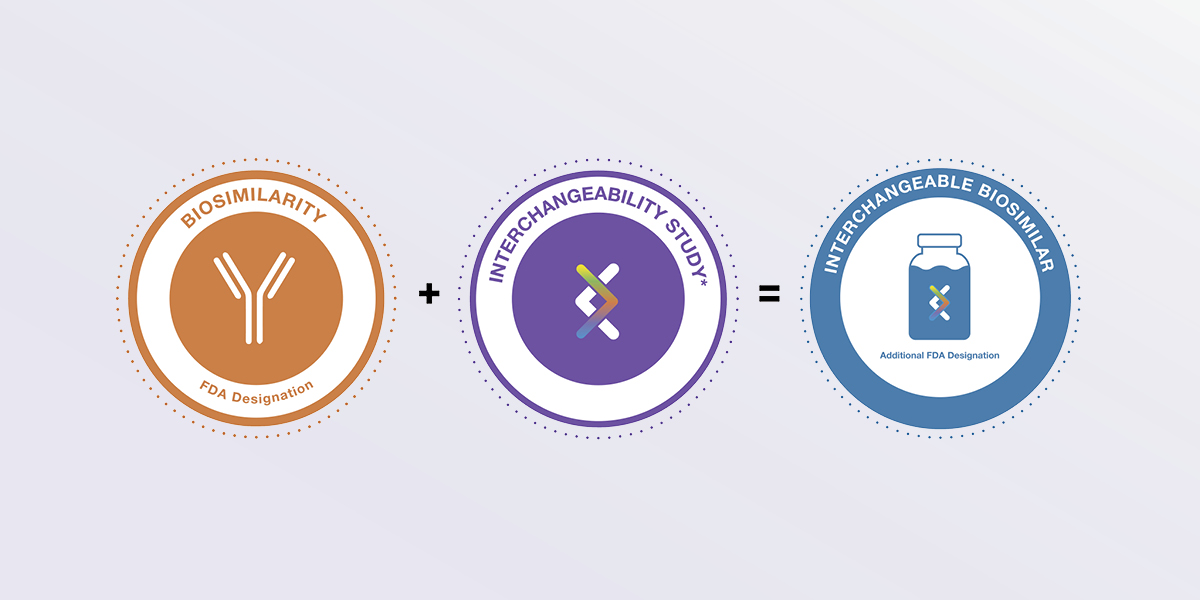
A biosimilar is a biological medicine proven to work in the same way as an approved reference biologic, with no clinically meaningful differences in terms of safety, potency and purity.1
Interchangeability is a distinct FDA designation. To attain it, a biosimilar must undergo an additional study*, called an Interchangeability Study, which shows how patients do when they are switched back and forth from the reference product to the Interchangeable candidate.1
Only when a biosimilar gains Interchangeable status from the FDA can it be called an Interchangeable biosimilar. It can then be automatically substituted at the pharmacy, depending on state laws.2
According to the FDA, prescribers and patients can expect that the Interchangeable product “will have the same clinical result as the reference product in any given patient.”2
*One or more studies may be required by the FDA to demonstrate Interchangeability.
References: 1. US Food & Drug Administration website. Biosimilar Product Regulatory Review and Approval. Accessed September 10, 2021. https://www.fda.gov/files/drugs/published/Biosimilar-Product- Regulatory-Review-and-Approval.pdf 2. US Food & Drug Administration website. Prescribing biosimilar and interchangeable products. Accessed September 9, 2021. https://www.fda.gov/drugs/biosimilars/prescribing-biosimilar-andinterchangeable-products
